OUR SCIENCE
A new generation of therapies
We apply scientific innovation and clinical intelligence to advance a new generation of differentiated therapies to better address unmet needs for patients with immunologic and inflammatory diseases.

OX40
(mAb)
Atopic dermatitis
Alopecia areata
BTK
(small molecule)
Rheumatoid arthritis;
Chronic spontaneous urticaria
IL-36R
(mAb)
Generalized pustular psoriasis;
Hidradenitis suppurativa
Novel Targets
To be disclosed
ILT7
(mAb)
To be disclosed
*Formed partnership with Aditum Bio to create Celexor Bio
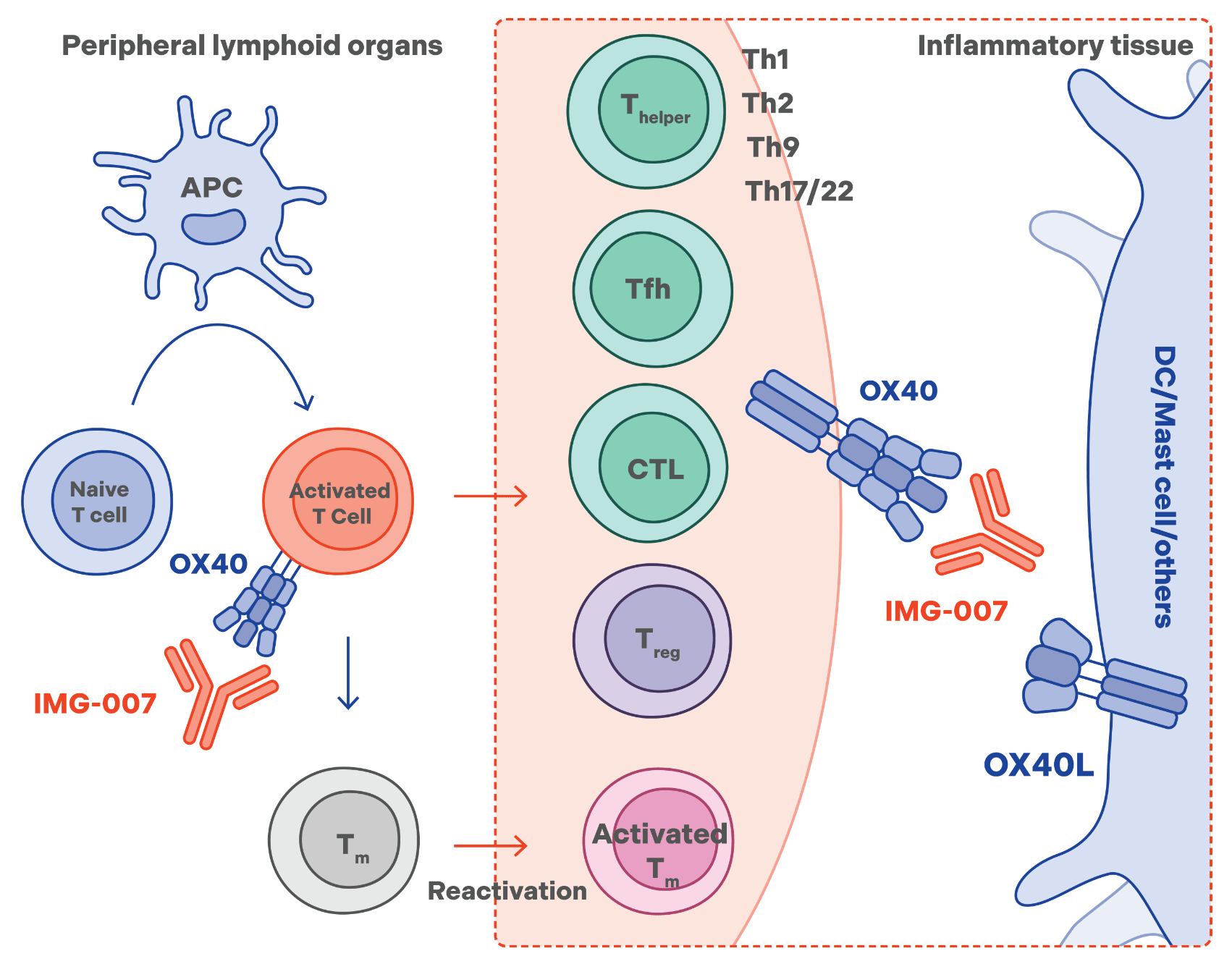
IMG-007
the first clinical-stage nondepleting anti-OX40 monoclonal antibody (mAb)
IMG-007 is a humanized immunoglobulin G (IgG1) mAb targeting OX40, a costimulatory receptor that presents primarily on activated T cells.1 Via bioengineering, IMG-007 has a silenced antibody-dependent cell-mediated cytotoxicity (ADCC) function to mitigate risks associated with ADCC-induced cytotoxicity, as well as a prolonged half-life to enable the potential for once-every-12-weeks (q12w) dosing.
In a phase 1 single-ascending dose (SAD) study in healthy adults, IMG-007 demonstrated a 31-day half-life at anticipated therapeutic doses and a favorable safety profile, without pyrexia or chills. IMG-007 is being evaluated in phase 2a studies for the treatment of moderate to severe atopic dermatitis (AD) and alopecia areata (AA).
IMG-007 clinical trials
IMG-007 is currently in two Phase2A clinical trials to evaluate its safety, pharmacokinetics, and efficacy in healthy adults.
Pharmacokinetics and efficacy in adult patients with moderate to severe AD (including patients who failed prior biologics)
Pharmacokinetics and efficacy in adult patients with AA with 50% or greater scalp hair loss
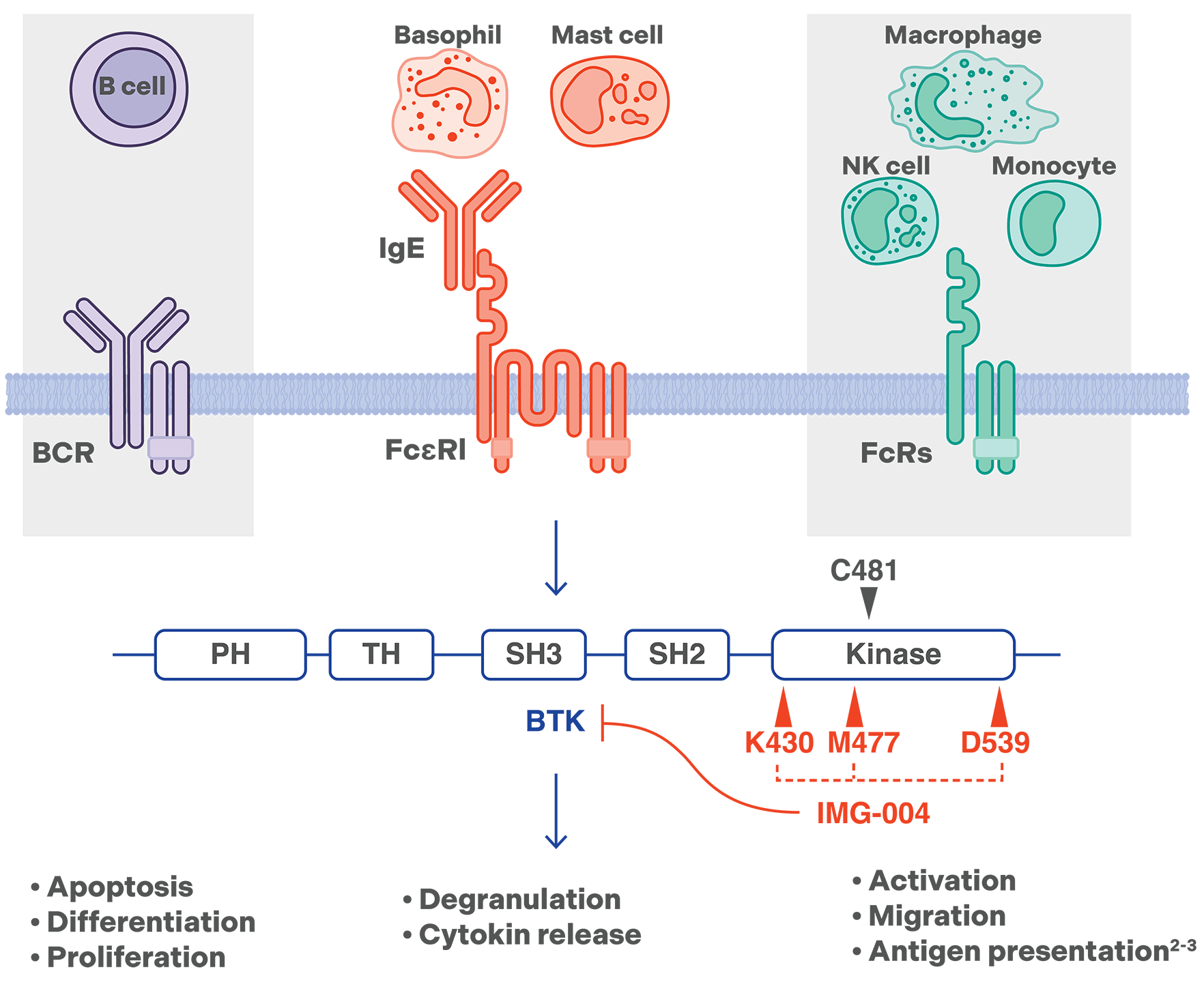
IMG-004
a potent, highly selective, noncovalent, reversible BTK inhibitor
Designed specifically for inflammatory and autoimmune diseases that usually require long-term treatment, IMG-004 is a noncovalent, reversible, potent, highly selective, and brain-permeable oral inhibitor of Bruton’s tyrosine kinase (BTK). In a phase 1 SAD study in healthy adults, IMG-007 showed a favorable safety profile, long half-life, and durable pharmacodynamic effect, potentially allowing for once daily (qd) dosing. It is currently being investigated in a phase 1 multiple-ascending dose (MAD) study in healthy adults.
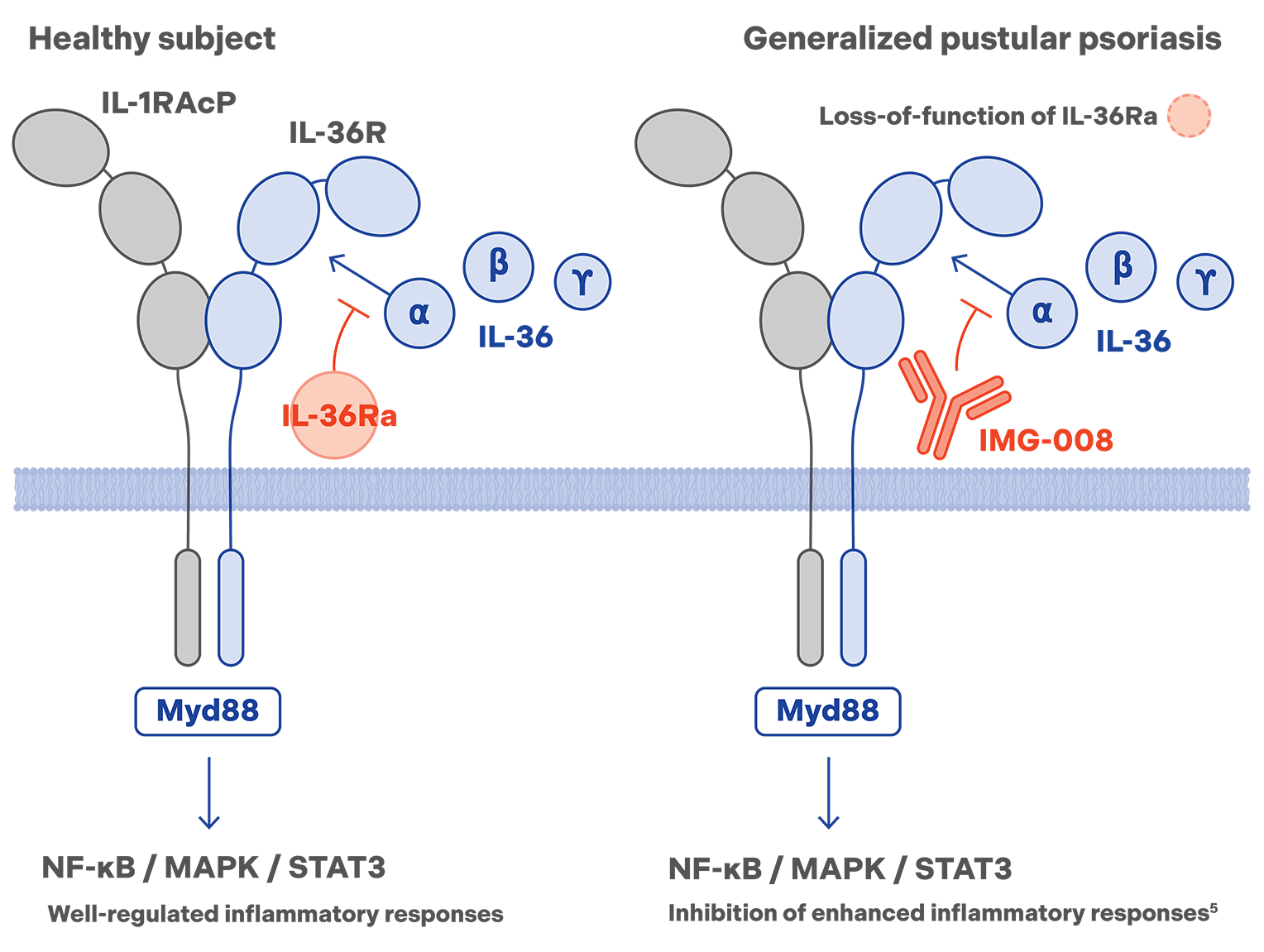
IMG-008
clinical-stage long-acting anti-IL-36R mAb
IMG-008, a discovered asset via our proprietary QuadraTek® platform, is an anti-IL-36R mAb, bioengineered to have a long half-life and enhanced exposure. It has the potential to provide effective and more convenient q12w dosing to treat generalized pustular psoriasis (GPP). Preclinical research indicates that IMG-008 has an approximately 4x longer half-life and 2x higher exposure, while retaining IL-36R blocking activity similar to an approved IL-36R mAb.4
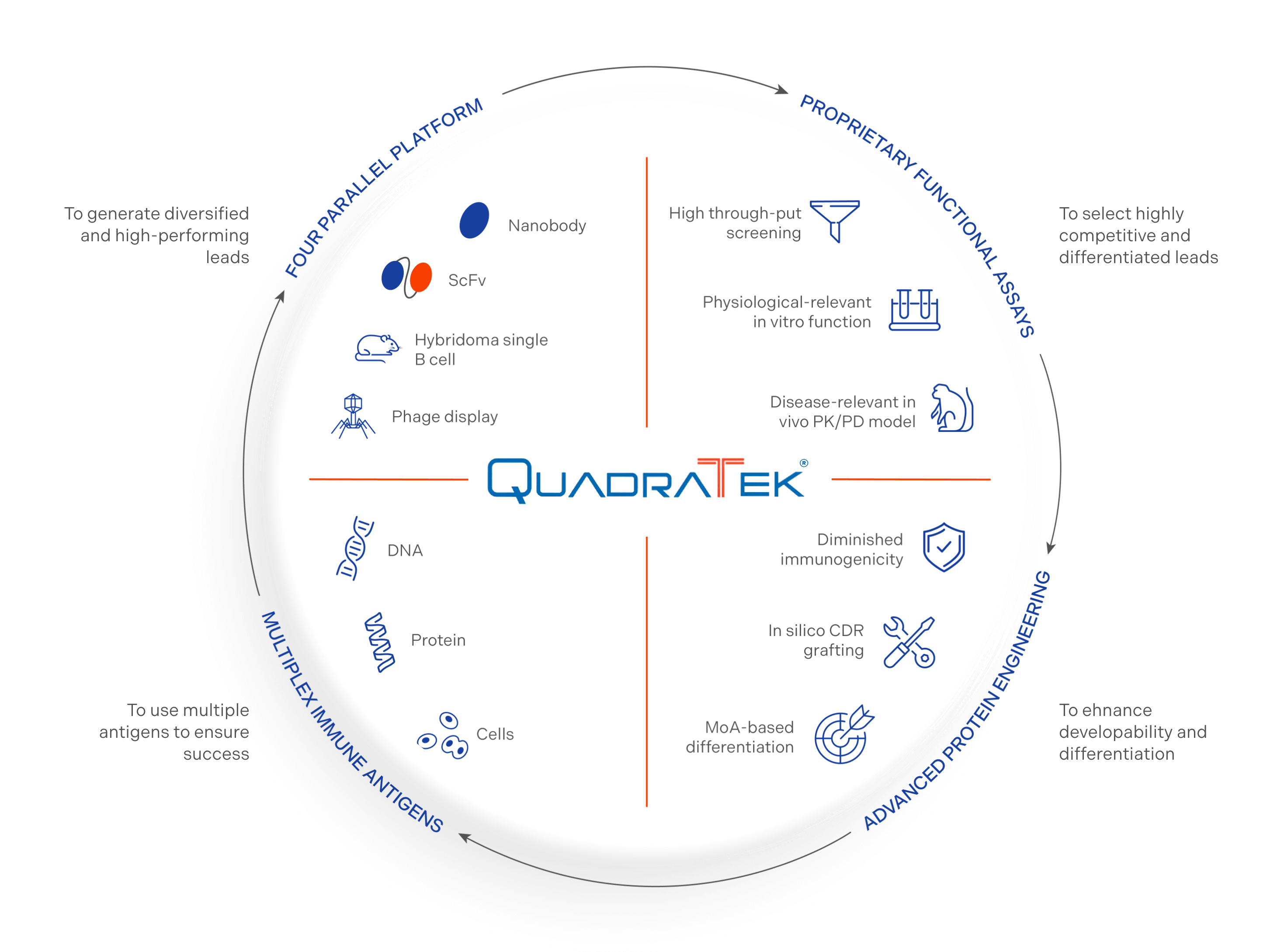
QuadraTek®:
a platform for innovation
Our proprietary QuadraTek® platform is designed to create highly differentiated biologic drug candidates with the potential to better address unmet needs in immunologic and inflammatory diseases.
Four parallel systems to generate diversified, high-performing leads
Proprietary functional assays to select competitive, differentiated drug candidates
Advanced protein engineering to optimize different aspects of a product
References
- Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134)[J]. Annual review of immunology, 2009, 28: 57-78.
- Garg N, Padron EJ, Rammohan KW, Goodman CF. Bruton’s tyrosine kinase inhibitors: the next frontier of B-cell-targeted therapies for cancer, autoimmune disorders, and multiple sclerosis. J Clin Med. 2022;11(20):6139. doi:10.3390/jcm11206139
- Neys SFH, Rip J, Hendriks RW, Corneth OBJ. Bruton’s tyrosine kinase inhibition as an emerging therapy in systemic autoimmune disease. Drugs. 2021;81(14):1605-1626. doi:10.1007/s40265-021-01592-0
- Data on file. Inmagene Bio.
- Sachen KL, Arnold Greving CN, Towne JE. Role of IL-36 cytokines in psoriasis and other inflammatory skin conditions. Cytokine. 2022;156:155897. doi:10.1016/j.cyto.2022.155897