OUR SCIENCE
A new generation of therapies
We apply scientific innovation to advance a new generation of differentiated therapies to better address unmet needs for patients with autoimmune and inflammatory diseases.

Imagene Pipeline
OX40
(mAb)
Atopic dermatitis
Alopecia areata
IMG-007
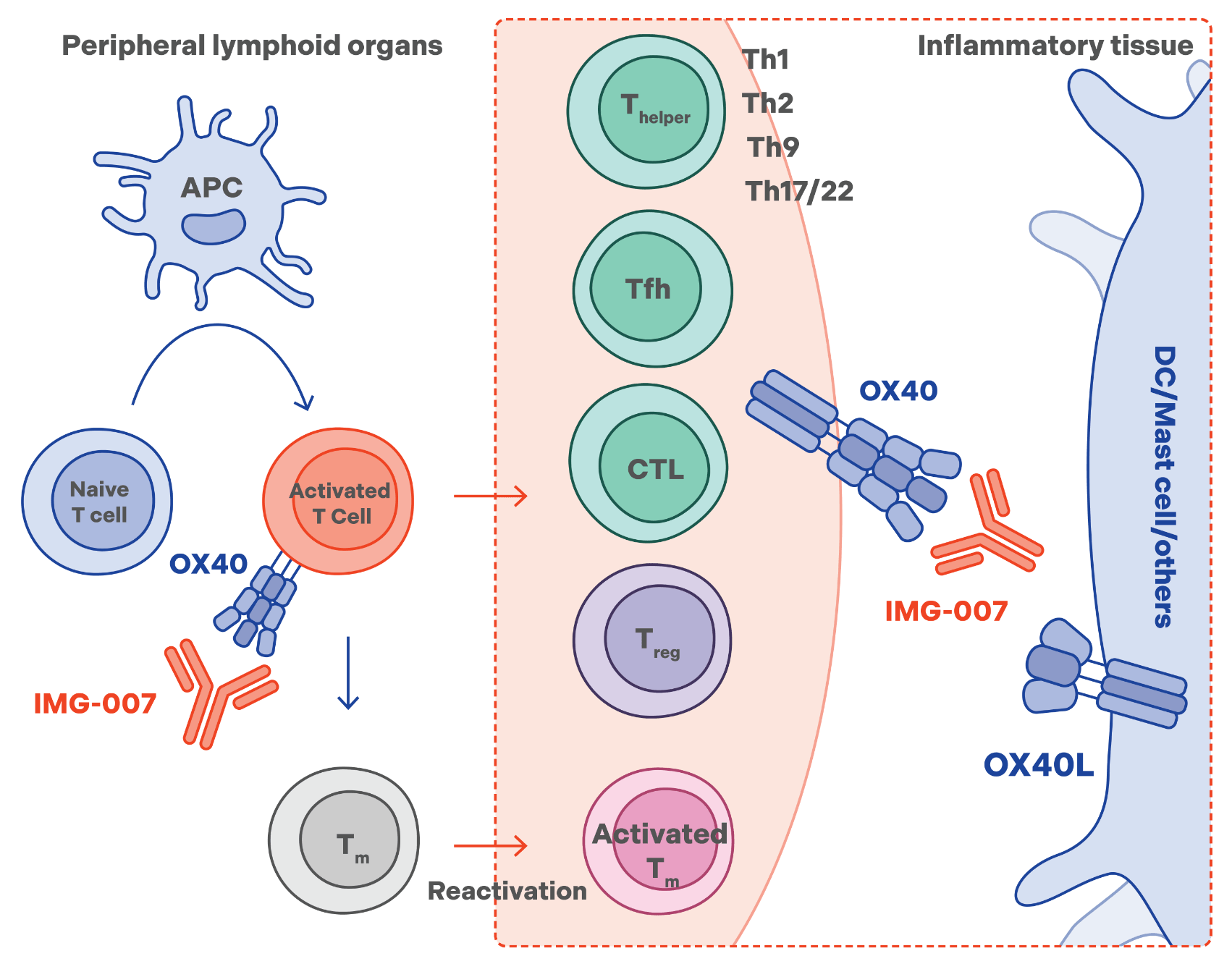
Our pipeline focuses on IMG-007, a novel non-depleting anti-OX40 mAb bioengineered with Fc N297A mutations to abolish antibody-dependent cellular cytotoxicity (ADCC). IMG-007 is designed to specifically bind to OX40 on activated T cells and block the interaction between OX40 and OX40L without killing the T cells. OX40-OX40L signaling is important in T cell activation, expansion, and survival, playing an important role in the pathogenesis of a spectrum of autoimmune and inflammatory diseases. Aberrant OX40-OX40L signaling can contribute to the development of these diseases by promoting excessive T cell activation and persistent tissue inflammation. Elevated OX40L and OX40 expressions have been observed in affected tissues across various autoimmune and inflammatory diseases, including but not limited to atopic dermatitis.
IMG-007 has been evaluated in atopic dermatitis (AD) and alopecia areata (AA).
- In a Phase 2a proof of concept study, moderate-to-severe atopic dermatitis patients treated with a four-week course of IMG-007 experienced marked, durable relief of skin lesions. In addition, durable inhibition of serum inflammatory markers of diverse T helper (Th) cells, including Th1, Th2 and Th17 cells, was observed. IMG-007 was well tolerated overall.
- In a separate Phase 2a proof-of-concept trial in patients with severe alopecia areata, four-week treatment with IMG-007 resulted in a dose-related clinical activity signal of hair regrowth and marked and durable inhibition of markers of Th1, Th2, and CD8+ T cells in the scalp tissue. IMG-007 was well tolerated.
A Phase 2b dose-finding study in patients with moderate-to-severe AD is currently ongoing.
IMG-007 clinical trials
IMG-007 is currently enrolling A Phase 2b Study Evaluating the Efficacy and Safety of IMG-007 in Adult Participants With Moderate-to-Severe Atopic Dermatitis (ADAPTIVE)
Pharmacokinetics and efficacy in adult patients with moderate to severe AD (including patients who failed prior biologics)